Researchers at the USA Department of Energy’s Oak Ridge National Laboratory (ORNL) have announced the development of a new battery technology which expands renewable energy use and captures airborne carbon dioxide (CO2).
The findings were published in the journal of Power Sources.
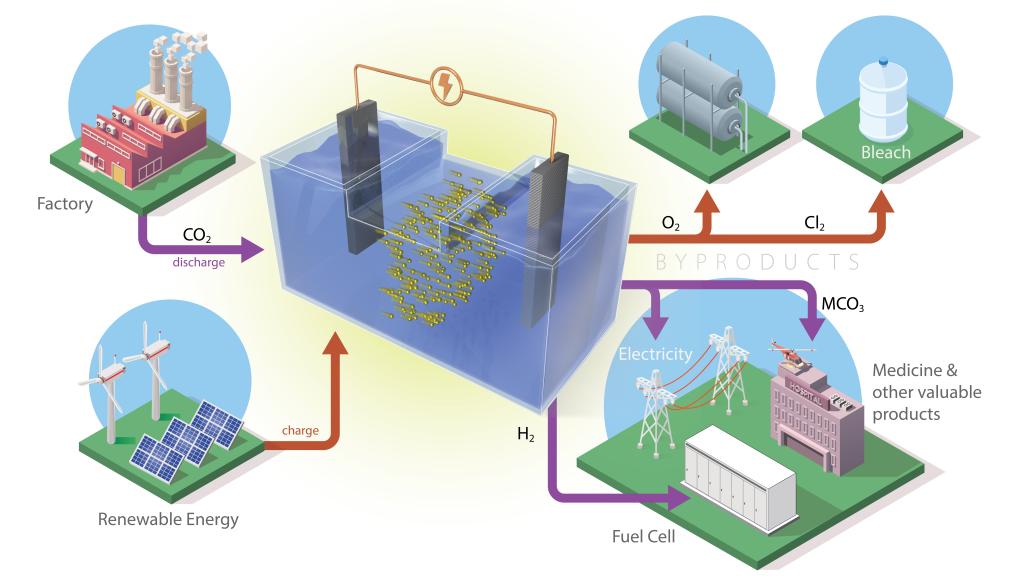
These batteries store energy generated by solar panels or wind turbines and use an electrochemical reaction to capture CO2 from industrial emissions, such as power plants, converting it into valuable products (pictured above).
ORNL researchers have created and tested two new battery types that convert CO2 gas into a solid form, which can potentially be used in other products. The aluminium-carbon dioxide (Al-CO2) battery was developed for long-term storage, and is capable of providing over 10 hours of electricity storage and operating for more than 600 hours without losing capacity.
It captures almost twice as much CO2 as the Na-CO2 battery and can operate in a single chamber, eliminating barriers to ion movement.
“The Transformation Energy Science and Technology (TEST) initiative at ORNL is essential for addressing climate change,” said Ilias Belharouak, an ORNL Corporate Fellow and initiative director. “Using free electrons to store CO2 and converting it into revenue-generating products is a concept I never imagined a decade ago, but this is just the beginning.”
The new ORNL battery types use different components to determine their operational lifetime, energy storage capacity, size, weight, and charging speed. One type combines CO2 with sodium from saltwater using an inexpensive iron-nickel catalyst, while the other combines CO2 with aluminium.
Both approaches use abundant materials and a saltwater electrolyte, making the batteries safer than existing technologies, according to lead researcher Ruhul Amin.
The battery design can also be tuned to produce more byproducts for industries like pharmaceuticals or cement. The only gases released are oxygen and hydrogen, which can be captured for energy or fuel production.
Amin’s team used specialised techniques to study the battery and found that altering the charge/discharge cycle could prevent film buildup, allowing the battery to be reactivated.
The challenge for the Al-CO2 battery is to scale up its operation, while for the Na-CO2 battery, the focus will be on developing a fine, dense, mechanically stable ceramic membrane to separate the battery chambers.
“Extending the operating lifetime and improving CO2 capture efficiency are our next steps,” Amin said.
[Image credit: Credit: Andy Sproles/ORNL, U.S. Dept. of Energy]